Participate in the EUP OHAMR survey to strengthen AMR research capacity!
We are launching a survey to identify gaps in strengthening research capacity in the field of AMR. Your input is valuable!
The survey aims to conduct a global assessment of the needs, barriers (incl. logistical, legal, financial), and potential collaboration opportunities for capacity strengthening activities that EUP OHAMR can support in the coming years.
The survey covers all areas related to AMR, as well as the three challenge-driven focal areas identified in the Strategic Research and Innovation agenda (SRIA) of the Partnership – to prevent, treat and control the spread of AMR.
Please fill in the survey by 23rd December 2025, 17:00 CET through the link below. The survey takes 20–25 minutes, can be completed anonymously, and you may provide your email for feedback. Please respond based on your experience.
Your input will help strengthen AMR research capacity within the EU and beyond and foster collaboration.
Contact us if you have any questions!
Austrian Agency for Health and Food Safety (AGES)
on behalf of the EUP OHAMR Work package 4
mail OHAMR-A4.1@ages.at

Successful kick-off in Brussels for the EUP OHAMR
The European Partnership on One Health AMR has now officially kicked off after two intense days of productive meetings, and insightful talks and discussions. Watch the recording from the external part of the event to learn more about the partnership!
The kick-off on 22-23 September gathered more than 100 participants on site in Brussels to outline our objectives, align our goals, meet the EUP OHAMR team and initiate the collaborative journey ahead. Another 200 people joined online for the external part of the event to learn more about the partnership and listen to talks and knowledge sharing sessions.
Thank you to the speakers, EUP OHAMR partners and all participants for making this such a successful event!
Programme
- Welcome and Introduction
- Katarina Bjelke, Director General, Swedish Research Council, Sweden
- Kasia Jurczak, Head of Unit Combatting Diseases, DG-RDT, European Commission
- AMR: setting the scene
- Dr. Hatim Sati, AMR expert, Medical Doctor, and Public Health Professional
- An overview of the EUP OHAMR programme
- Overview of the partnership – mission, vision, scopes and functions; Shahida Syed, EUP OHAMR Head of Secretariat
- Panel discussion with Work package leads/representatives: How do we achieve our intended outputs, outcomes and impact?; Barbara Junker, WP2, DLR, Germany; Sophie Gay, WP3, ANR, France; Giorgio Vingiani, WP4, APRE, Italy; Linda van Gaalen, WP5, ZonMw, Netherlands; Francesca Hodges WP6, UKRI (MRC), United Kingdom
- Insight forum: Delivering impact through global collaboration
- Panel discussion with experts: Shawon Lahiri, moderator; Charu Kaushic, Scientific Director/Institute of Infection and Immunity (CIHR), Canada; Henning Gädeke, Chair, The European & Developing Countries Clinical Trials Partnership (EDCTP3); Helle Engslund Krarup, Director of operations, International Centre for Antimicrobial Resistance Solutions (ICARS), Hatim Sati, AMR expert; Louise Norton-Smith, Director of External Affairs, CARB-X
- Concluding remarks
- Shahida Syed, EUP OHAMR Head of Secretariat

Panel members of the Insight forum.

Kick-off for the EUP OHAMR!
We are very pleased to invite you to the live stream of the kick-off for the European Partnership on One Health Antimicrobial Resistance.
Date: 23 September 2025
Time: 13.00 – 16:20 CEST
Live streamed from Brussels, Belgium
This session outlines our strategic vision, key objectives, and roadmap for the collaborative journey ahead. Learn more about the partnership and listen to diverse viewpoints on shaping effective and enduring global partnerships in the AMR space to create lasting impact.
To receive the video link to the event, please register below. We look forward to your participation!
Draft programme
13:00 – 13:15 Welcome and Introduction
13:15 – 13:45 AMR: setting the scene
- Dr. Hatim Sati, AMR expert, Medical Doctor, and Public Health Professional
13:45 – 15:10 An overview of the EUP OHAMR programme
- Overview of the partnership – mission, vision, scopes and functions (20’)
- Panel discussion with Work package leaders: How do we achieve our intended outputs, outcomes and impact? (50’)
- Q&A (15’)
15.10 – 15:30 Coffee break
15:30 – 16:15 Insight forum: Delivering impact through global collaboration
- Panel discussion with experts
16:15 – 16:20 Concluding remarks

Voices on EUP OHAMR
What are your expectations for the new partnership and what should it accomplish? What are the most important priorities in AMR research? Hear the voices of Dame Sally Davies, Irene Norstedt, Jan-Ingvar Jönsson, Till Bachmann and Sabiha Essack on the EUP OHAMR.
At the JPIAMR Final Conference, that took place on 7-8 May in Stockholm, Sweden, we asked Jan-Ingvar Jönsson, Chair of JPIAMR, and Till Bachmann and Sabiha Essack from the JPIAMR Scientific Advisory Board to share some key achievements of JPIAMR and their wishes and expectations for the new partnership.
We also met with Prof. Dame Sally Davies, Special Envoy on AMR for the UK, and Irene Norstedt, Director, DG Research and Innovation, European Commission, to hear their thoughts on AMR research prioritisation, existing knowledge gaps in the AMR field and the importance of One Health and international collaboration in the EUP OHAMR.
Watch the interviews through the link below!

Interviews from the JPIAMR Final Conference (youtube.com/jpiamr)
Link to another websiteWatch the interviews with Jan-Ingvar Jönsson, Till Bachmann, Sabiha Essack, Irene Norstedt and Dame Sally Davies.

JPIAMR Final Conference 7-8 May 2025
Link to another websiteLearn more about the conference that highlighted the impact of 10 years transnational AMR research and marked the the milestone of the JPIAMR transitioning to EUP OHAMR.

Research & Innovation call 2026
The first EUP OHAMR joint transnational call will be within the focus area of providing innovative and cost-effective treatment options. The call will be launched in November 2025.
Focus area: Provide innovative and cost-effective treatment options
Grant form: Project grant
Call opens: November 2025
Estimated budget: Approx. 35 million Euros
The potential research objectives for this call will be as outlined in the EUP OHAMR Strategic Research and Innovation Agenda:
- To develop new antimicrobials, novel treatment protocols or alternative treatment strategies against Human Infectious diseases along with their respective diagnostics.
- To improve, preserve and reinforce the clinical efficacy of the current treatment antimicrobials.
- Identification of barriers to access, availability, quality and uptake of therapeutic solutions and development of solutions to overcome those barriers.
- Assessment and prediction of the impact of economic incentives and regulations on drug development, drug production, drug supply and treatment availability.
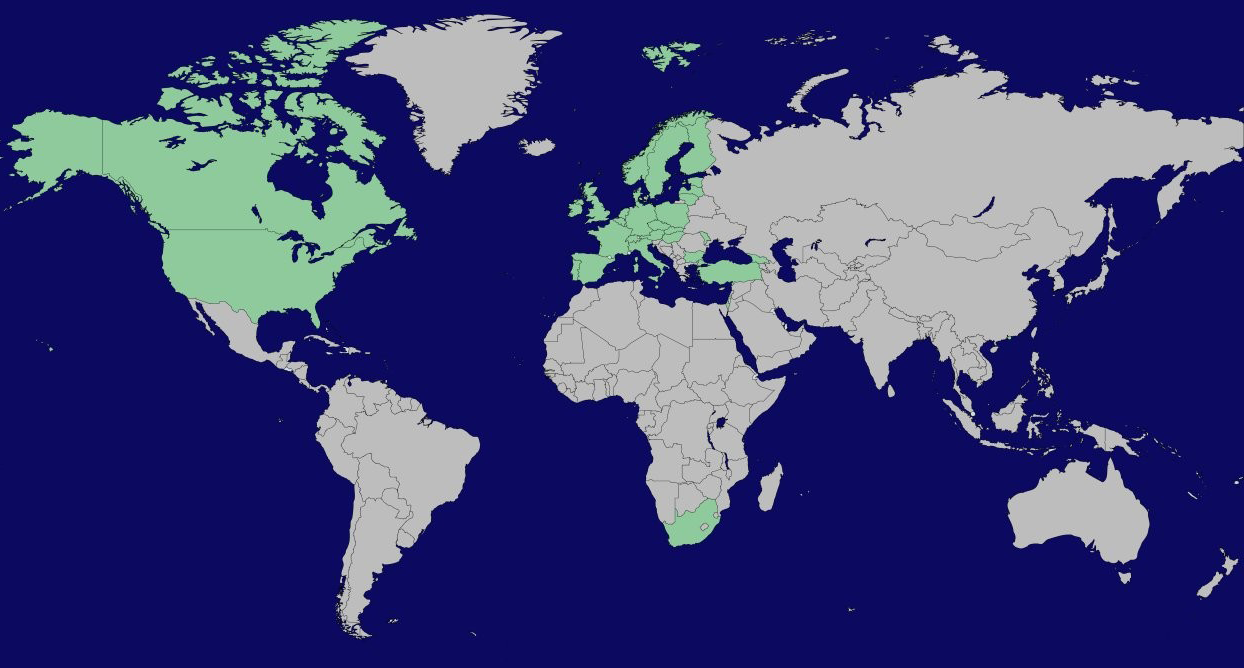
Participating countries
Austria, Belgium, Canada, Czech Republic, Denmark, Estonia, Finland, France, Germany, Hungary, Ireland, Israel, Italy, Latvia, Lithuania, Malta, Moldova, Netherlands, Norway, Poland, Portugal, Slovakia, South Africa, Spain, Sweden, Switzerland, Turkey and United Kingdom

Welcome to EUP OHAMR!
After years of preparations, The European Partnership on One Health Antimicrobial Resistance has now started.
The partnership brings together 53 organisations across 30 countries from EU and beyond that together with the European Commission will provide joint support to boost One Health AMR Research and Innovation (R&I) in all its dimensions: coordinating joint transnational calls, actions to strengthen the capacity of the AMR research community, provide frameworks for alignment of policy and stakeholder engagement, and tools to valorise the new knowledge for the benefit of citizens and society as a whole.
Shahida Syed, Head of EUP OHAMR Secretariat:
“It is truly exciting that EUP OHAMR has begun! Through this partnership, we are fostering cross-sector research collaborations to drive the innovation needed for real-world impact. Together, we can find the instruments to understand and curb antimicrobial resistance on a global scale and develop and sustain life-saving treatments and tools.”
The EUP OHAMR in brief
- Consortium of 53 organisations across 30 countries
- 10 year programme 2025-2035
- Ambitious framework of programmes consisting of funding for R&I projects, capacity strengthening, knowledge translation, data exploitation and impact
- Approx. €250 million pooled funding for R&I projects
- Co-funded by the EU with up to €75 million towards the implementation of the programme and support for R&I projects
- Coordinated by the Swedish Research Council, Sweden