Joint transnational call 2026
This first EUP OHAMR joint transnational call for projects will explore new treatments to tackle AMR. The call "Treatments and adherence to treatment protocols", involves 37 funding organisations from 28 different countries with an estimated total call budget of over 31 million Euro. The call is co-funded by the European Union.
Call summary
Closed 2026
| Call title | Treatments and adherence to treatment protocols |
| Focus area | Provide innovative and cost-effective treatment options |
| Grant form | Project grant |
| Grant period | 3 years |
| Call opens | 18 November 2025 |
| Call closes | 2 February 2026 (pre-proposals) |
| Call budget | Over 31 million Euros |
Please note
- Only transnational research and innovation projects will be supported.
- An online partner search tool is available.
- Applications must be submitted to the EUP OHAMR submission platform and follow the EUP OHAMR pre-proposal/full proposal template.
- Applications to EUP OHAMR joint transnational calls can require the submission of additional information on national funding platforms.
- All applicants must have fulfilled both joint and national requirements for an application to be eligible.
- Applicants are strongly advised to confirm their funding eligibility with their national/regional funding organisation before submission.
- For updates to the call text, please see the History of change section at the bottom of this page.
Aim of the call
Drug-resistant infections are responsible for an increasing number of treatment failures, increased mortality and decreased food productivity. Inappropriate use, poor adherence to prescriptions, and overuse of antibiotics are one of the main drivers of AMR and have a detrimental impact on the effectiveness of these critical medicines.
To develop novel treatment protocols or alternative treatment strategies against infectious diseases and to improve, preserve and reinforce the clinical efficacy of the current treatment antimicrobials is vital. It is also necessary to identify barriers preventing the proper adherence of the end-users to the treatment protocols already in place.
This first EUP OHAMR Call aims to improve the treatment success rates of the patients/animals/plants affected by bacterial or fungal infections by providing new treatment options while reducing the risk of resistance in the different One Health settings.
Call topics
Research & innovation proposals submitted under this call must address one of the following topics:
Topic 1
Identify and develop new combination treatments using existing or innovative antimicrobials or antimicrobial with adjunctive treatments to extend drug efficacy and combat resistance.
Resistance limits the usability of many commonly-used antibiotics and antifungal agents in Human Health, Animal Health, and Plant Health.
Proposals addressing this topic should identify and develop therapies to be used in combination (combination of different antimicrobials, or combination of an antimicrobial and a non-antimicrobial that improves activity or facilitates a better targeting towards the site of infection) to reduce the development of resistance against antibacterial and antifungal treatments and extend the usability of inexpensive and readily available antimicrobials. These studies should be underpinned by scientific rationale and mechanism of action of these treatments.
In the framework of this topic, improvement of existing combination treatments is eligible (i.e. pharmacokinetics and pharmacodynamics, mode of administration). The choice of the targeted pathogens should be well justified. For the proposals having a Human Health interest, the proposed combination treatment should be directed against one of the bacterial or fungal pathogens included in the WHO priority lists:
- WHO bacterial priority pathogens list, 2024 (who.int)
- WHO fungal priority pathogens list to guide research, development and public health action (who.int)
Topic 2
Develop tools and methods to improve adherence to treatment protocols.
A low adherence to the treatment protocols by end-users (patients, farmers, citizens) leads to a decreased probability of success and to an increased risk of resistance to antibacterial and antifungal treatments.
Proposals addressing this topic should identify the reasons of poor adherence to treatment protocols (Human, Animal, Plant), and/or develop innovative tools (including digital tools) and methods (including sociological and behavioural approaches) to improve the adherence to treatment protocols and/or test and compare the efficiency of existing or innovative tools and methods on the adherence to treatment protocols.
Engagement with end-users is mandatory. The consideration of vulnerable groups, which often have reduced access to conventional health and care services, is expected.
Topic 3
Assess the impact of antimicrobials for veterinary and agricultural use on the risk of AMR transmission to humans and the environment to inform policies on the restriction of some antimicrobials for human use.
Proposals addressing this topic are expected to assess the impact of mechanisms of action, formulations, routes of administration and treatment regimens of antibacterial and antifungal drugs authorized for veterinary and agricultural use on the risk of emergence and transmission of AMR to humans and the environment.
Proposals addressing this topic should also aim to improve the formulation, dosage, delivery, routes of administration and treatment regimens (including pharmacokinetics and pharmacodynamics) currently used in the veterinary and agricultural sector, to decrease the risk of cross-resistance, or transmission to humans and the environment.
The aim is to generate evidence to support policies that restrict certain antimicrobials for exclusive human use and inform policies such as the WHO List of Medically Important Antimicrobials (who.int).
Requirements for all call topics
The following requirements applies for all call topics:
- Proposals should consider how the proposed approach will impact the risk of resistance in other One Health settings, and how the proposed approach could be extended to other One Health settings.
- Proposals should explain their feasibility by outlining realistic objectives, practical methodologies, and achievable timelines (e.g.: workplan, risk identification, allocation of resources, capacity of the consortium to conduct the work, business plan if the proposal envisages commercial component, etc).
- Proposals must clearly demonstrate the potential health, social and/or economic impact(s) of the expected results. In particular, the proposals should explain how the uptake of the expected project results by the society/ end-users/ next actors in the value chain will be facilitated.
- Proposals should clearly demonstrate the benefit of working together and the unique contribution of each partner (i.e. expertise, resources). In addition, the proposals should demonstrate the added-value of transnational collaboration: sharing of expertise and resources (models, databases…), harmonization of data, access to innovative technologies, etc.
- Participation of the private sector (start-up, SMEs, industry) in the consortium is encouraged for all call topics if appropriate and if allowed according to national funding eligibility rules.
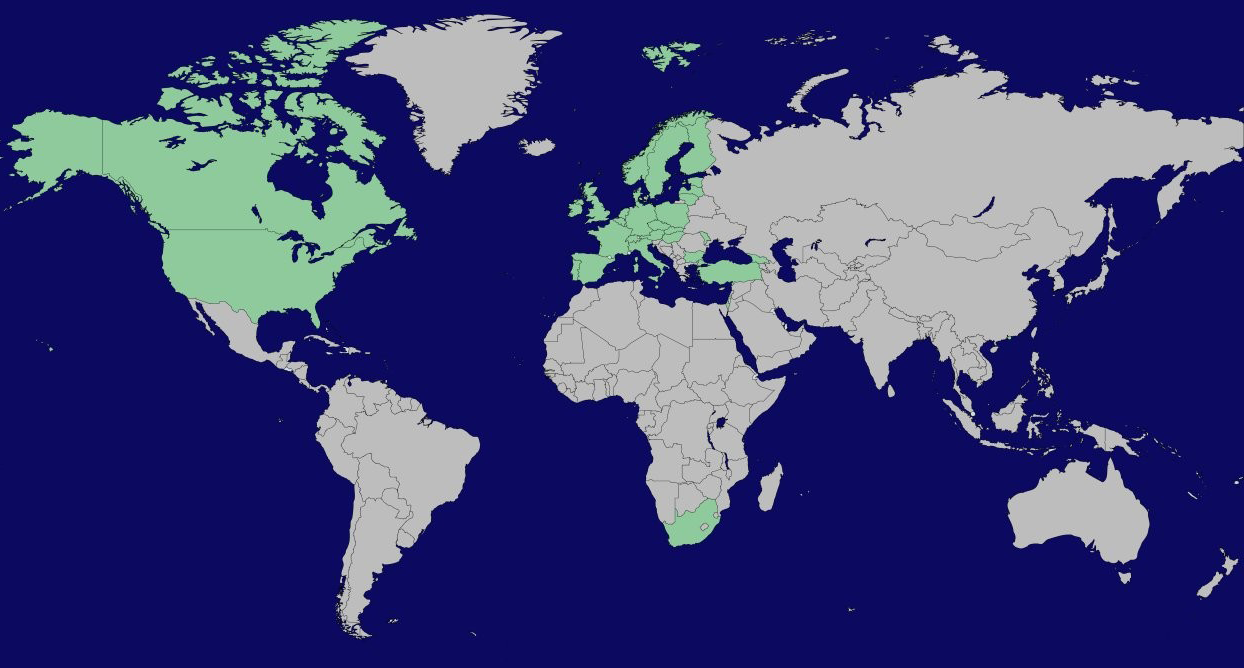
Participating countries and funding organisations
There are 37 funding organisations from 28 different countries participating in this call with a total budget of over 31 million Euro.
- Austria, Fonds zur Förderung der Wissenschaftlichen Forschung (FWF)
- Belgium, Belgium Fonds de la Recherche Scientifique (FNRS)
- Belgium, Fonds Wetenschappelijk Onderzoek-Vlaanderen (FWO)
- Belgium, Service Public de Wallonie (SPW)
- Canada, Canadian Institutes of Health Research (CIHR)
- Czech Republic, Ministerstvo Zdravotnictvi Ceske Republiky (MZCR)/ Agentura pro zdravotnicky vyzkum (AZVCR)
- Denmark, Innovationsfonden, Innovation Fund Denmark (IFD)
- Estonia, Sihtasutus Eesti Teadusagentuur (ETAG)
- Finland, Suomen Akatemia (AKA)
- France, Agence Nationale de la Recherche (ANR)
- Germany, Bundesministerium für Forschung, Technologie und Raumfahrt (BMFTR)/ Deutsches Zentrum für Luft- und Raumfahrt Projektträger (DLR-PT)
- Hungary, Nemzeti Kutatási, Fejlesztési Innovációs Hivatal (NKFIH)
- Ireland, Department of Agriculture, Food and the Marine (DAFM)
- Ireland, Taighde Éireann – Research Ireland (TÉ-RI)
- Ireland, The Health Research Board (HRB)
- Israel, Ministry Of Health – Chief Scientist Office (CSO-MOH)
- Italy, Fondazione Regionale per la Ricerca Biomedica (FRRB)
- Italy, Ministero della Salute (MOH-IT)
- Latvia, Latvijas Zinatnes Padome (LZP)
- Lithuania, Lietuvos Mokslo Taryba (LMT)
- Malta, Xjenza Malta (XM)
- Moldova, National Agency for Research and Development (NARD)
- Netherlands, Nederlandse Organisatie voor Wetenschappelijk Onderzoek (NWO)
- Norway, The Research Council of Norway (RCN)
- Poland, Narodowe Centrum Nauki (NCN)
- Portugal, Fundacao para a Ciencia e a Tecnologia (FCT)
- Slovakia, Centrum Vedecko Technickych Informacii Slovenskej Republiky (CVTI SR)
- South Africa, South African Medical Research Council (SAMRC)
- Spain, Instituto Aragones de Ciencias de la Salud (IACS)
- Spain, Agencia Estatal de Investigación (AEI)
- Spain, Instituto de Salud Carlos III (ISCIII)
- Sweden, Vetenskapsrådet, Swedish Research Council (SRC)
- Switzerland, Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung (SNSF)
- Turkiye, Turkiye Bilimsel ve Teknolojik Arastirma Kurumu (TUBITAK)
- United Kingdom, Medical Research Council – United Kingdom Research and Innovation (MRC – UKRI), Department of Health and Social Care (DHSC)
- United Kingdom, Innovate UK – United Kingdom Research and Innovation (Innovate UK – UKRI)
National rules and requirements
The funding will be provided by the national/regional research funding organisations participating in the call. Each of them will fund their national/regional applicants, according to their own eligibility rules.
To be eligible for funding, applicants must comply with the regulations and scientific remits of their own funding organisations as detailed in the national and regional requirements or specific regulations of their corresponding funding partner organisation, see link below.
Applicants are strongly advised to confirm their funding eligibility with their national/regional funding organisation before submission.
General conditions for application
The call will only support transnational research and innovation projects. You can find more information on the rules for composing your consortium in the section Eligibility/Composition of the transnational consortium.
- The call will follow a two-step evaluation process: Submission of a pre-proposal; successful consortia will be invited to submit a full proposal.
- Pre-proposals and full proposals must be written in English, and must follow the format and the guidelines provided in the pre-/full proposal template. Pre-proposals or full proposals that do not follow the template guidelines (i.e. length of the different sections, number of CVs and letters of intent) will be rejected without further review.
Overlaps between applications and grants
Proposals submitted to this call should not present overlap with proposals submitted to/granted by other Horizon Europe or EU4Health calls or with projects previously funded by JPIAMR.
In their full proposal, applicants will be asked to identify potential related projects and describe how their proposal does not overlap, or possibly complements, a related EU project (including, for European Partnerships such as the Partnership on Animal Health and Welfare (eupahw.eu), projects submitted to external calls, as well as research activities funded internally).
Application procedure
Pre-proposals and full proposals must be submitted by the project coordinator on the EUP OHAMR on-line submission platform. No other means of submission (i.e. post or e-mail) will be accepted. Please note that some funding organisations might request an additional mandatory submission on their own national/regional platform. Pre-/full proposals submitted on a national/regional platform but not on the EUP OHAMR submission platform will be rejected without further review.
Applicants must respect the submission deadlines indicated. Submission of pre-proposals or full proposals after the submission timeline will not be accepted.
Applicants must consider enough time to complete the information on-line. In particular, please consider that collecting some information, such as the Participant Identification Code (PIC) of your research organisation, may require external procedure. Applicants should also ensure that they will have a proper internet connection at the time of submission.
Call timeline
- 18 November 2025 (11.00 CET): Call opens
- 26 November 2025 (14.00 CET): Webinar for applicants
- 2 February 2026 (13.00 CET): Deadline pre-proposals
- 29 April 2026: Communication of the results of the pre-proposal assessment
- 17 June 2026 (13.00 CEST): Deadline full proposals
- 30 October 2026: Final funding recommendation announced to applicants
- November 2026: Publication of the results on the EUP OHAMR Website during the AMR Awareness Week
- December 2026 – April 2027: Projects start
Eligibility
Funding recipients
Joint research proposals may be submitted by partners belonging to the following categories:
- Academia (public and private universities, other higher education institutions or research institutes).
- Clinical/public health sector (hospitals/public health and/or other health care settings and health organisations, including primary health care).
- Enterprises (private companies of all sizes involved in research and innovation).
- Operational stakeholders – e.g. patient advocacy organisations, municipalities and local governments, local/national NGOs. Operational stakeholders should be able to provide useful knowledge to the consortium, ensure the consortium’s research is useful and translatable to their (or other) organisational contexts, and/or influence decision making or create change within their organisations. Operational stakeholders should be engaged in the research process from conception of the study to dissemination.
Each partner institution will be represented by one (1) Principal Investigator who will be the researcher scientifically responsible for the implementation of tasks assigned.
Please note
According to national/regional regulations, certain categories may not be eligible for funding by a specific funding organisation, or some categories may be mandatory.
Conflict of interest
To avoid conflicts of interest, employees of the EUP OHAMR partner organisations are not allowed to apply to this call, with the exception of the staff working in AGES, ISCIII, IACS, TUBITAK and DLR.
AGES has been excluded from the call preparation. Firewall measures (such as organisation of the work in separate divisions) have been put in place internally in ISCIII, IACS, TUBITAK and DLR to guarantee an equal treatment of the applicants and applications. In particular, the persons working on the call implementation will ensure that no confidential information will be shared with their research community.
Composition of the transnational consortium
To be eligible, the submitted pre-/full proposals must respect the following eligibility rules regarding the composition of their consortium. Consortia not respecting the eligibility rules will be rejected without further review.
- The consortium must include a minimum of three (3) eligible partners asking for funding from three (3) different eligible countries (including at least two amongst EU Member States or Associated Countries).
- The consortium can include a maximum of six (6) project partners, including non-funded partners.
- The maximum number of partners can be increased to seven (7) if the consortium includes:
- at least one partner from an under-represented country (For the purpose of this call, the under-represented countries are: Czech Republic, Estonia, Hungary, Latvia, Lithuania, Malta, Moldova, Poland, and Slovakia) or
- at least one partner where the Principal Investigator meets the definition of an Early-Career Researcher or
- a start-up, SME, or an Industry.
- A consortium cannot include more than two (2) partners requesting funding from the same funding organisation. Please note that for United Kingdom partners, the limit of two applies to both UK funding organisations, meaning that the consortium cannot include more than two UK partners funded either by MRC-UKRI or by Innovate-UK.
- A principal investigator can coordinate only one (1) submitted pre-proposal/ full proposal.
Please note
- Hungarian legal entities affected by the Council Implementing Decision (EU) 2022/2506 are eligible to apply to calls for proposals as non-funded partners (i.e. without requesting or receiving funding from the Call).
- A consortium cannot include, as funded partners as well as non-funded partners, any legal entities established in Russia, Belarus, or in non-government-controlled territories of Ukraine, or any legal entities established outside Russia but whose proprietary rights are directly or indirectly owned for more than 50% by a legal person, entity or body established in Russia. This list is not extensive and may evolve.
- For more information on other restrictive measures, consult the general annexes of the 2023-2025 Horizon Europe funding programme (ec.europa.eu)
Letter of intent
At both the pre- and full proposal stages, all partners, including non-funded partners, must submit a signed letter of intent along with their pre-/full proposal (the templates to be used for the letters of intent are included in the pre-/full proposal templates).
In the absence of these letters, the proposal will be declared ineligible. At the pre-proposal stage, the letter of intent must be signed by the project investigators representing the different partner institutions. At the full proposal stage, the letter of intent should also be signed by a person having the legal mandate to represent the partner institutions.
Early Career Researcher definition
For the purpose of this call, an Early-Career Researcher (ECR) is a PhD holder, up to 8 years after the year of PhD award, holding a position at a recognised institution.
The 8-year period may be extended to allow for career breaks including documented parental leave, positions of trust in trade union organisations and student organisations, mandatory military or civil service, illness (own illness or care for close family members), medical internships or medical fellowship (applies to clinically active professionals). The last two categories may involve periods of up to 24 months each.
Please note that the EUP OHAMR definition of an ECR may differ from the national/regional definition.
Non-funded partners
Project partners not eligible for funding (e.g. from countries not participating in the call or not fundable according to national/regional regulations of the funding partner organisations) may be involved in projects if they bring their own funding.
The following rules and requirements apply for non-funded partners:
- The budget of non-funded partners must be included in the proposal (In-Kind Budget) and shall not exceed 30% of the requested total transnational project budget.
- The number of non-funded project partners in a consortium must not exceed the number of funded partners.
- A project partner not receiving funding cannot be the coordinator of a proposal.
- Non-funded partners have the same responsibilities as funded partners, i.e. they must accept all EUP OHAMR rules and guidelines as set out in this call text.
Modification of the composition of the transnational consortium
The composition of the consortium should not be modified between the pre- and the full proposal except for:
- widening (see next section)
- in case of force majeure/unforeseen event (e.g. change of professional affiliation, lab relocation, prolonged absence of the Principal Investigator, etc.)
- upon recommendation of the Peer Review Panel or request of the Call Steering Group
Please note
Changes in the composition of the consortium must be approved by the board of funders organising the call ahead of the submission of the full proposal.
The Principal Investigator representing the coordinating research institution must send an e-mail to the Joint Call Secretariat, and to the respective funding organisations, at least 15 calendar days before the full proposal submission deadline to confirm the eligibility of the proposed modifications.
Widening process
Consortia which are invited to the second stage of the call (full proposal) will be able to increase their initial size by adding one (1) new partner eligible for funding by an under-subscribed funding organisation. An under-subscribed funding organisation is a funding organisation that may underspend the full amount of funds it has committed to the call.
Consortium coordinators will be notified of this option in their invitation letter to submit a full proposal. The list of under-subscribed funding organisations will be included in the full proposal template. Please note that the total number of partners in the consortium must not exceed eight (8).
The widening process promotes inclusiveness, ensure global participation, relevance and impact of the submitted projects in and outside Europe, as well as maximisation of the use of the committed resources.
Financial modalities
Funding is initially granted for a maximum of three years in accordance with national/regional regulations and applicable legal provisions.
Project partners will be funded by their relevant national/regional funding organisation. Therefore, eligible costs, funding rules and the type of studies allowed will vary between the respective funding organisations. Thus, it is recommended that each project partner defines their own budget in accordance with the funding rules of their own country/region. Please note that some funding organisations may limit the number of national/regional applicants per proposal or may limit the maximum budget national/regional applicants can request within a consortium.
Any budget change between the pre-proposal and the full proposal must be approved by the national/regional funding organisations before submission, provided that:
- it complies with the national/regional rules;
- the oversubscription of the funding organisation does not surpasses the threshold;
- the newly requested budget still complies with the eligibility rules regarding the composition of the consortium, and;
- the changes are duly justified and related to the scope and ambition of the proposal.
Please note that if a partner is found to be non-eligible at any step of the process by one of the funding organisations, the entire proposal could be rejected without further review.
Social and gender equity, cultural sensitivity and economic viability
It is important that consortia and research proposals are founded upon principles of social and gender equity, cultural sensitivity and economic viability. Consortia are highly encouraged to apply these principles to the composition, leadership and management of research projects. Especially where Low- and Middle-income countries are involved in the proposal, the impact to improving health and well being should be considered.
Where relevant, research projects are expected to apply an intersectional and multi-dimensional approach by integrating sex, gender and other individual and population-level determinants of health (such as age, socio-economic status, ethnicity) into the project’s design, implementation, monitoring, evaluation and knowledge translation activities.
Research projects are expected to consider individual- and population-level determinants of health when collecting and analysing data to design and/or implement interventions in ways that are accessible and affordable to target beneficiaries, to systematically capture and report on sex, gender, and other relevant factors in the project research outputs, and to meaningfully engage the participation of targeted marginalised groups in the research activities.
Evaluation process
This call will follow a two-step evaluation process that will comply with the principles of transparency, equal treatment and non-discrimination. Your application is evaluated in competition with the other applications by an international peer review panel (PRP).
The process can be summarised as follows:
- Formal eligibility check of the pre-proposals by the Joint Call Secretariat (JCS) and the national/regional funding organisations.
- Evaluation (written assessments and grading) of pre-proposals by independent reviewers. Each application is evaluated by three reviewers.
- First peer review panel meeting where the pre-proposals are discussed and ranked.
- Board of funders meeting where it is decided which pre-proposals are invited to submit a full proposal. The decision is based on the reviewer’s recommendations and the balance between requested and available national/regional budgets.
- Formal eligibility check of the full proposals by the Joint Call Secretariat (JCS) and the national/regional funding organisations.
- Evaluation (written assessments and grading) of full proposals by independent reviewers. Each application is evaluated by three reviewers.
- Second peer review panel meeting where the full proposals are discussed and ranked.
- Full proposals recommended for funding by the PRP and selected for funding by the board of funders undergoes an ethics review by an Ethics Panel. Only those proposals approved by both the scientific evaluation and ethics assessment (complying with all central Horizon Europe and regional/national ethical requirements) are funded.
- The funders takes their funding decision, based on the ranking lists established by the PRP, the available funding and the Ethics panel recommendations.
Applicants can appeal against the evaluation outcome if they suspect a breach in the implementation of the evaluation and selection procedures. This redress procedure only covers the procedural aspects of the evaluation. You will find more information on the redress procedure through the link below.
Responsibilities of the grantees
There are terms and conditions for your grant regarding setting up a project consortium agreement, Open Science, reporting, ethics and communication. As an applicant you must keep in mind these obligations while drafting your budget and planning your workload. Read more on the page Responsibilities of the grantees.
General Data Protection Regulation (GDPR)
By submitting an application, the applicants consent to the use, processing and retention of their personal data in accordance with article 6.1 (e) and (c) of the General Data Protection Regulation (GDPR) (2016/679) and for the purposes of:
- processing and evaluating the pre- and full proposal where processing shall be lawful only if and to the extent that processing is necessary for the performance of a task carried out in the public interest or in the exercise of official authority vested in the controller;
- administering any subsequent funding award;
- managing the relationship between the applicant and the Funding Partner Organisations;
- analysing and evaluating the call;
- providing aggregate data to national and European surveys and analyses on the funded projects;
- complying with audits that may be initiated by the Funding Partner Organisations and the European Commission (or its agencies).
In addition, by submitting an application (pre- and full proposal) to this call, the applicants agree to share their personal data with Funding Partner Organisations based outside the European Economic Area and with third parties such as evaluators (some of which may be based outside the European Economic Area) in relation to the above activities.
The following Funding organisations outside the European Economic Area will use their national data protection rules:
- Canada (CIHR)
- Israel (CSO-MOH)
- Moldova (ANCD)
- South Africa (SAMRC)
- Switzerland (SNSF)
- Turkey (TUBITAK)
- United Kingdom (UKRI)
By the time of the call launch, the European Commission issued adequacy decisions for personal data protection laws in Israel, Switzerland and the United Kingdom.
Funding organisations and third parties may link the data that applicants provide in the application with national, bibliographic or external research funding data which is available through public subscription-based databases (e.g. Scopus, Web of Science, etc.) or other national/open datasets.
Confidentiality
The content of the pre-proposals and full proposals received under this Joint Call is deemed to be confidential, except for consortia including project partners applying for funding from the Swedish funding organisation (SRC). For those consortia, the applications (pre- and full proposals) may be made available upon request after the respective call deadlines.
Responding to a EUP OHAMR call for proposals, both as project coordinator or project partner, gives the EUP OHAMR, the European Commission and the Funding Partner Organisations the right to use and store the information submitted for analysis of the call success rate, national response rate, etc.
The proposals will be handled confidentially by the JCS and by the national/regional funding organisations. In selecting the international experts for the PRP, the JCS shall endeavour to avoid any possible conflicts of interest. Each expert will sign a declaration of confidentiality and confirm the absence of conflicts of interest. In case of a conflict of interest the reviewer will be withdrawn from evaluating the respective proposal. Conflicts of interest are managed and recorded throughout the evaluation process.
Accepting a EUP OHAMR grant award and associated grant contract from a national funding organisation gives the EUP OHAMR, the European Commission and Funding Partner Organisations the right to store, share, and analyse information on beneficiaries and consortia (rules may differ between different countries). Composition of the awarded consortia (Principal investigators, Institution) as well as the title, acronym and abstract of funded projects will be published and openly accessible. No data will be shared with third parties or commercial entities without the formal consent of the project coordinators, except for consortia including project partners applying for funding from the Swedish Funder (SRC). For those consortia, the applications (pre- and full proposals) may be made available upon request after the respective call deadlines.
Contact
All questions related to the general eligibility rules and general evaluation process should be addressed to the EUP OHAMR Joint Call Secretariat (JCS), which is hosted by the French National Research Agency (ANR).
All questions related to the national/regional eligibility rules and national/regional eligibility costs should be addressed to the national/regional funding organisations.
The only official communication line between the project consortia and the JCS is the project coordinator. Throughout the application procedure the JCS will only contact by e-mail the project coordinators, who must forward all information to other partners of their consortia. This includes evaluation results.
EUP OHAMR Joint Call Secretariat
Yue Xiao and Sophie Gay, French National Research Agency
+33 1 73 54 82 41/ +33 1 78 09 80 39
History of change
Any changes to the call text after 18 November 2025 will be noted here.
2026-01-14, Pre-proposal form updated as follows:
- Letter of Intent template (p. 11-12): References to “Annex B of the Call text” has been replaced with “the Responsibilities of the Grantees awarded in the JTC1 framework, as described in the call text”
- Letter of Intent template (p. 11-12): References to “Section 11 of the Call text” has been replaced with “the section of the Call text referring to the General Data Protection Regulation (GDPR)”